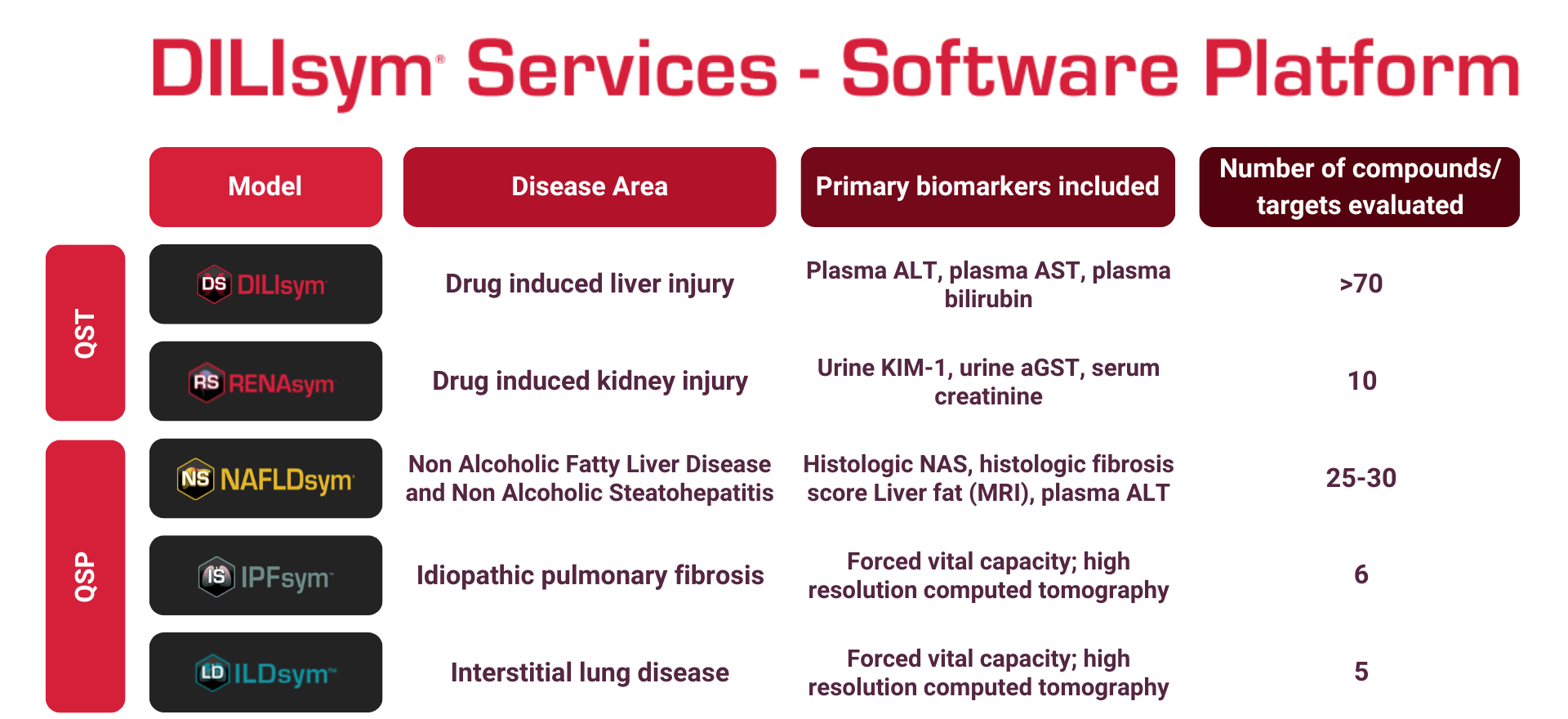
DILIsym Services, Inc. was incorporated in 2014 and operates in Research Triangle Park, North Carolina. DILIsym Services, Inc. is committed to providing the pharmaceutical industry with the tools, resources and information to more efficiently develop safe and effective drug therapies that increase the availability of vital therapeutics for the patients who need them. DILIsym Services, Inc. has developed the DILIsym® modeling software and the NAFLDsym® modeling software to support these objectives. DILIsym Services, Inc. makes this software available to pharmaceutical companies in addition to performing services projects with the modeling software. In these projects, the DILIsym Services, Inc. expert scientific team engages clients from initial program design through data input and results interpretation.
Our mission is to help ensure the safety of drug therapies by leveraging our expertise in the areas of drug-induced liver injury, metabolic diseases, and modeling and simulation. We aim to make drug development more efficient for the pharmaceutical industry by informing key decisions, which will in turn lead to increased availability of vital medicines for the patients who need them.
DILIsym® Services may be utilized by companies involved in the research and development of drugs or chemicals, including services such as conducting simulations of drug-liver interactions, and designing computer software for companies to be used in modeling liver responses to a drug or chemical.